Clinical Services
University Pathology Laboratory
The UPL, accredited by the College of American Pathologists (CAP), was established to provide cutting edge technologies for diagnosis and management of human diseases.Click here for latest UPL brochure
Detection and Genotyping of Human Papilloma Virus (HPV)
Digene HPV Test using Hybrid Capture II
The Digene HPV Test using Hybrid Capture II is a FDA approved signal amplified hybridization antibody capture microplate assay that utilizes chemiluminescent detection. Target HPV DNA in the specimen will hybridize with specific HPV RNA probe cocktail in the kit and the resultant RNA:DNA hybrids are captured onto the surface of a microplate well coated with antibodies specific for these hybrids.
At present, the UPL provides reflex HPV detection test for women diagnosed with atypical squamous cells of undetermined significance (ASCUS) in their cervical cytology. HPV detection for cervical cancer screening and monitoring for recurrence of disease can also be provided.
HPV Genotyping by PCR and DNA Sequencing
UPL provides HPV Genotyping by PCR and DNA Sequencing for quality control of DNA-based HPV detection tests from referral laboratories. Other technologies for HPV genotyping including HPV arrays are being developed.
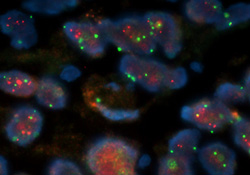
HER-2 Fluorescent in Situ Hybridization
PathVysion®, the only FDA approved HER-2 assay,is performed by UPL for assessment of amplification status of the HER-2 oncogene. The assessment aids with the selection of patients suitable for adriamycin-based therapy and of patients who may respond to Herceptin® treatment. The test can be performed on formalin fixed paraffin embedded surgical pathology tissue blocks.

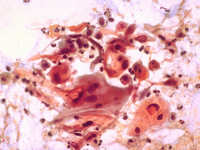
HKU Cervical Cytology Screening Laboratory
Since the establishment of the HKU Cervical Cytology Screening Laboratory in 1992, the laboratory is dedicated to the continual improvement of the quality of cervical cytology screening. Nearly 1.2 million samples have been reported with an average of 80,000 cases per annum.
The HKU Cervical Cytology Screening Laboratory is a pioneer in the introduction of state-of-the-art technologies:
- In March 2000, liquid-based cytology technology approved by the US FDA was adopted for full-scale cervical cancer screening.
- In 2001, the laboratory became the first laboratory in Hong Kong accredited by the College of American Pathologists. The accreditation status has been maintained since through rigorous bi-annual inspections by CAP.
- In 2004, a pilot study on HPV testing for triage of women with atypical squamous cells of undetermined significance (ASCUS) in cervical smears was conducted with the generous support of the SK Yee Medical Foundation. The impressive results encourage the adoption of a reflex HPV test for women with cervical cytology diagnosed with ASCUS.
- In July 2005, the Laboratory was the first in Hong Kong and Asia to introduce the latest model of automated cervical cytology screening imager approved by the US FDA.


Tissue Processing and Reporting Laboratory
The tissue processing and reporting laboratory provides tissue processing service for surgical biopsy specimens. This includes tissue processing, embedding, microtome-sectioning, and H&E staining. The pathologists on duty would perform macroscopic description, block-sampling of specimens such as cervical loop excision, as well as pathology reporting. Similar services are also available for contract research.Tissue Microarray is the construction of a paraffin embedded block, comprised of multiple tissue elements derived from individual “donor” tissue blocks. It allows cost effective conduction of immunohistochemistry and in-situ hybridization. It can be applied for clinical and translational research including clinical trials.
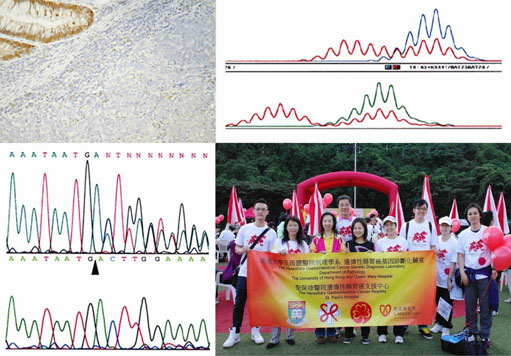
Hereditary Gastrointestinal Cancer Genetic Diagnosis Laboratory
The Hereditary Gastrointestinal Cancer Genetic Diagnosis Laboratory was established since 1995. We are currently providing genetic tests, genetic counseling, psychosocial support and advice and referral for prophylactic screening for families at risks for the Lynch (also known as Hereditary Nonpolyposis Colorectal Cancer, HNPCC), Familial Adenomatous Polyposis (FAP) and other types of Polyposis syndromes. This is a charitable service supported by the Hong Kong Cancer Fund, aiming to achieve colon cancer prevention in local high risk families through genetic testing and appropriate prophylactic screening. Since 2006, we have gained support from St. Paul’s Hospital that allows us to set up a charitable patient referral centre in their hospital venue to facilitated population-wide patient recruitment. The Laboratory works in collaboration with all public and private doctors in Hong Kong to provide a comprehensive genetic diagnosis service, so as to help them to plan appropriate prophylactic screening for at risk individuals. Over the years, these have resulted in hundreds of polyps or early cancers being removed and thus saving lots of lives. More importantly, with our service, a lot of individuals from these at risk families are proven not inherited the cancer-predisposing mutations, and thus leads in great savings on medical resources and relief of psychological burden. To date, over 1000 families has benefited from our genetic diagnosis service. Amongst these, 263 families were confirmed to carry HNPCC, APC, PJS, JP, PTEN or TP53 gene germline mutations, with predictive genetic testing done for over 1180 family members. The laboratory has generated a large database on mutation spectrum of DNA mismatch repair gene in Chinese population, and uncovered a novel mechanism causing HNPCC through EPCAM gene deletion, the latter has become a standard genetic test worldwide. These have resulted in numerous high profile publications of both local and global importance, in prestigious journals including Nature Genetics and American Journal of Human Genetics. Our laboratory has provided the innovative model for academic researchers to serve the community through partnership with charitable organization, public and private health care providers.